 |
 |
AbstractA previously healthy, 3-year-old boy presented to the emergency department with an afebrile focal motor seizure. He was found crying and having a seizure 30 minutes earlier. During this seizure, he was jerking his head and right extremities. Subsequent magnetic resonance imaging showed acute infarction in the bilateral frontal lobes, chiefly in the left. After hospitalization, conventional angiography demonstrated bilateral stenosis of the distal internal carotid arteries with development of lenticulostriate collaterals, which confirmed the diagnosis of moyamoya disease. It is vital to recognize focal motor seizures and situations related to hyperventilation in children with a seizure, which imply a structural lesion and a provoked cerebral ischemia in preexisting moyamoya disease, respectively.
IntroductionMoyamoya disease (MMD) is an idiopathic vasculopathy characterized by progressive occlusion of bilateral, distal internal carotid arteries and consequent development of lenticulostriate collaterals. MMD accounts for 6% of childhood ischemic strokes [1], and is more common in East Asia [2]. The most common presenting symptom of MMD is hemiparesis [2]. However, 11%ŌĆÆ27% of children, especially toddlers, present with seizure [3-7]. Diagnosis of MMD should be expedited in the emergency department (ED) because surgical revascularization reduces the risk of stroke from 80% to 4% [1]. This report describes a 3-year-old boy with MMD who presented with a focal motor seizure possibly provoked by hyperventilation.
CaseA 3-year-old boy with an ongoing seizure was brought to the ED. During the seizure, his head was jerking to the left, the eyes were deviating upward, and the right extremities were jerking (Supplementary video 1, https://www.pemj.org/). This lateralizing sign suggested a structural lesion in the left frontal lobe. Although he was initially alert and crying, he became unresponsive as the seizure progressed. There was no immediate history of febrile illness, head injury, intoxication or vaccination.
Thirty minutes earlier, shortly after falling asleep, he was found having a seizure and crying. Until the arrival at the ED, he had undergone a total of 3 ictal episodes, each lasting 1ŌĆÆ4 minutes, with incomplete recovery of consciousness between the episodes. Given the frequent recurrences within 30 minutes, he was considered to have status epilepticus, and received intravenous lorazepam (0.1 mg/kg) and fosphenytoin (20 mg phenytoin sodium equivalent/kg).
He was born as a monozygotic twin and otherwise healthy except one episode of febrile seizure, which occurred 2 years earlier during a fight. His mother and twin brother had a history of febrile seizure. No family history was found on epilepsy, brain tumor, and childhood stroke.
The initial vital signs were as follows: blood pressure, 137/85 mmHg; heart rate, 118 beats per minute; respiratory rate, 22 breaths per minute; temperature, 36.3┬░C; oxygen saturation, 100% on room air; and blood glucose, 96 mg/dL. He weighed 18 kg and was alert and age-appropriately oriented, but he had slurred speech. Neurological examination was limited because of the sedation with intravenous lorazepam and fosphenytoin. Physical examination was otherwise unremarkable.
Initial laboratory evaluation showed a white blood cell count of 10.6 ├Ś 109/L with 38.3% neutrophils, 53.5% lymphocytes, and 5.2% monocytes. Venous blood gas analysis indicated acute respiratory alkalosis with a concomitant metabolic acidosis with the following values: pH, 7.44; PCO2, 31.9 mmHg; and HCO3, 22.4 mEq/L. Other laboratory findings were normal. Brain magnetic resonance imaging showed a high signal intensity in the bilateral frontal lobes, predominantly in the left, and a loss of flow voids in the bilateral middle cerebral arteries, suggesting acute infarction due to MMD (Fig. 1A-C). He was hospitalized in the pediatric neurology unit.
On day 1, he was found having right hemiparesis involving the arm and leg with muscle strength of 4/5 and right central facial palsy. An aspirin regimen (5 mg/kg/d) was initiated. Electroencephalography without a provocation maneuver indicated interhemispheric dysfunction. On day 4, magnetic resonance angiography showed bilateral stenosis of the distal internal carotid arteries (Fig. 1D). Conventional angiography confirmed MMD on day 5 (Fig. 2). No findings indicative of hypercoagulability, vasculitis or metabolic disease were found. Echocardiography was normal. On day 8, he was discharged with slightly improved hemiparesis (muscle strength of 4+/5) and facial palsy, after consulting with the neurosurgery department for revascularization. After discharge, he was found to have RNF213 single nucleotide polymorphism with heterozygote c.14429G>A (p.R4810K) variant. A few days after the presentation, his monozygotic twin brother was brought to the ED for having a seizure, and found to have MMD with the same genetic feature.
DiscussionIt is vital to recognize focal motor seizures and situations related to hyperventilation in children who present at the ED with a seizure. The former implies a structural lesion in the contralateral frontal cortex, and the latter predisposes children with preexisting vasculopathy to cerebral ischemia [8]. In the present case, the recognition of a focal motor seizure prompted neuroimaging, and excessive crying might have incurred or aggravated the cerebral ischemia.
In MMD, a focal motor seizure indicates a localizing sign. Seizures as a symptom of childhood ischemic stroke tend to be focal and accompanied by hemiparesis [9], and 68% of seizures in patients with MMD were focal [3-7]. Despite the relatively high frequency of focal seizures in MMD, it may be difficult to recognize a focal seizure and perform neuroimaging in toddlers who present at the ED with a seizure. Focal seizures are rarely witnessed, and account for the relatively low proportion of seizures in the ED (approximately 5%ŌĆÆ11%) [10,11]. Toddlers who have a focal seizure with fever can be misdiagnosed as having a ŌĆ£febrile seizureŌĆØ owing to the high frequency of febrile seizures (range, 2%ŌĆÆ5% of all children)[10]. Sedation requirement may contribute to the reluctance in performing neuroimaging.
In this case, some other features are notable. Situations related to hyperventilation lower PCO2, provoking cerebral vasoconstriction [3,8]. A possible provocation of cerebral ischemia by crying may be supported by the temporal relation between the crying, the ictal episodes and the detection of acute respiratory alkalosis at presentation. It is worth noting that crying is a common provoking event in toddlers with MMD [3]. The boy was younger than the median age (6ŌĆÆ8 years) of children with MMD [12,13]. The age younger than 3 years is associated with seizures as a presenting symptom of childhood ischemic strokes, such as MMD [3,9,14]. A prospective study shows the association of the age younger than 3 years and poor prognosis of MMD [15]. The boyŌĆÖs postictal hemiparesis that persisted longer than ToddŌĆÖs paralysis, which typically resolves within 48 hours, implied that the stroke was the cause of seizure [16]. The RNF213 heterozygote c.14429G>A (p.R4810K) variant, which is strongly associated with MMD in East Asia, was detected [17]. This genetic feature suggests that family history may facilitate the diagnosis of MMD, especially in East Asia.
Children with MMD can present with a focal motor seizure, and situations related to hyperventilation can provoke cerebral ischemia in such children. Recognition of these features in the ED may streamline the diagnosis of MMD, a treatable cause of childhood ischemic stroke.
Fig.┬Ā1.Magnetic resonance imaging and angiography findings showing acute infarction and bilateral stenosis of the distal internal carotid arteries and middle cerebral arteries (MCAs). Axial diffusion-weighted image shows high signal intensity in the bilateral frontal lobes, predominantly in the left (A). There are low apparent diffusion coefficient values in the corresponding areas, which are suggestive of acute infarction (B). Loss of flow voids in the bilateral MCAs on a coronal T2-weighted image indicates diffuse stenosis of the arteries (C). Time-of-flight magnetic resonance angiography shows bilateral stenosis of the distal internal carotid arteries, and occlusion of the bilateral M1 segments of MCA and A1 segments of the anterior cerebral artery (D). 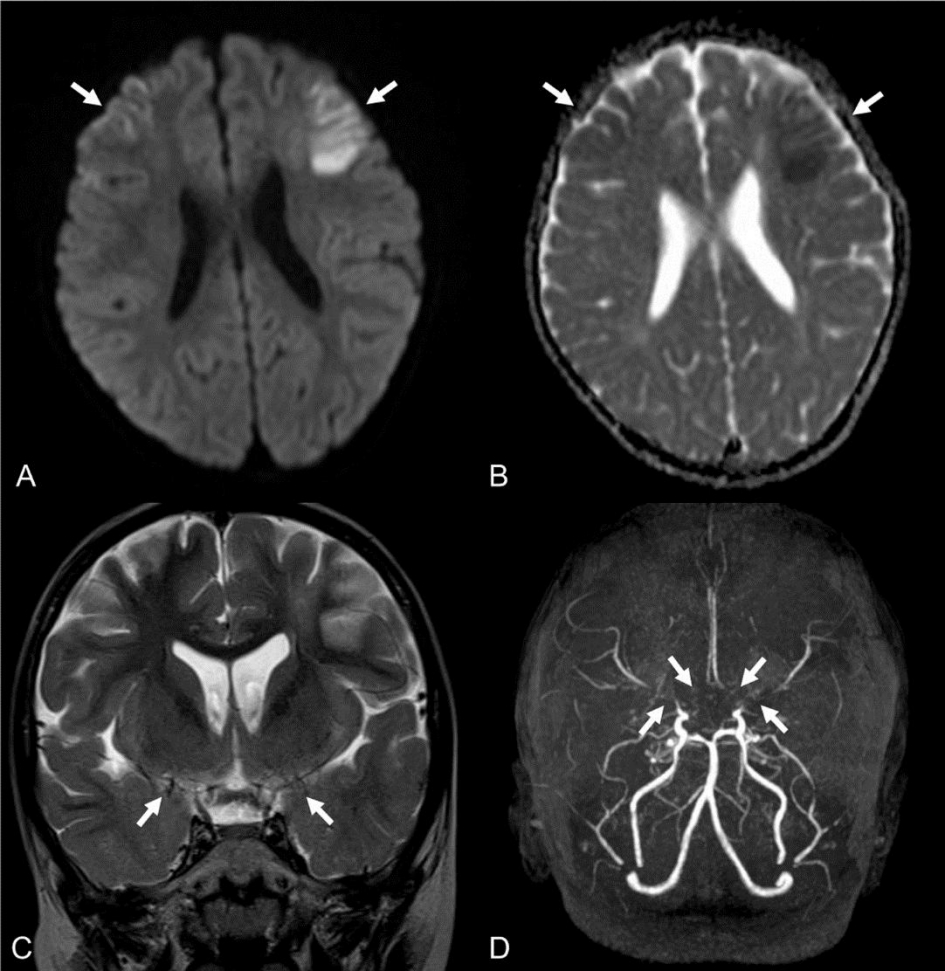
Fig.┬Ā2.Conventional digital subtraction angiogram showing bilateral stenosis of distal internal carotid arteries (black arrows, A and B) and leptomeningeal collaterals characterized by ŌĆ£puff of smokeŌĆØ appearance (arrowheads, A and B). Note that the stenosis of M1 segment is more severe in the left middle cerebral artery than in the right (white arrows, A and B). 
References1. Smith ER. Structural causes of ischemic and hemorrhagic stroke in children: moyamoya and arteriovenous malformations. Curr Opin Pediatr 2015;27:706ŌĆō11.
3. Kim YO, Joo SP, Seo BR, Rho YI, Yoon W, Woo YJ. Early clinical characteristics according to developmental stage in children with definite moyamoya disease. Brain Dev 2013;35:569ŌĆō74.
4. Nakase H, Ohnishi H, Touho H, Miyamoto S, Watabe Y, Itoh T, et al. Long-term followup study of "epileptic type" moyamoya disease in children. Neurol Med Chir (Tokyo) 1993;33:621ŌĆō4.
5. Mikami T, Ochi S, Houkin K, Akiyama Y, Wanibuchi M, Mikuni N. Predictive factors for epilepsy in moyamoya disease. J Stroke Cerebrovasc Dis 2015;24:17ŌĆō23.
6. Amlie-Lefond C, Ellenbogen RG. Factors associated with the presentation of moyamoya in childhood. J Stroke Cerebrovasc Dis 2015;24:1204ŌĆō10.
7. Ma Y, Zhao M, Zhang Q, Liu X, Zhang D, Wang S, et al. Risk factors for epilepsy recurrence after revascularization in pediatric patients with moyamoya disease. J Stroke Cerebrovasc Dis 2018;27:740ŌĆō6.
8. Tagawa T, Naritomi H, Mimaki T, Yabuuchi H, Sawada T. Regional cerebral blood flow, clinical manifestations, and age in children with moyamoya disease. Stroke 1987;18:906ŌĆō10.
9. Abend NS, Beslow LA, Smith SE, Kessler SK, Vossough A, Mason S, et al. Seizures as a presenting symptom of acute arterial ischemic stroke in childhood. J Pediatr 2011;159:479ŌĆō83.
10. Chen CY, Chang YJ, Wu HP. New-onset seizures in pediatric emergency. Pediatr Neonatol 2010;51:103ŌĆō11.
11. Martindale JL, Goldstein JN, Pallin DJ. Emergency department seizure epidemiology. Emerg Med Clin North Am 2011;29:15ŌĆō27.
12. Rafay MF, Armstrong D, Dirks P, MacGregor DL, deVeber G. Patterns of cerebral ischemia in children with moyamoya. Pediatr Neurol 2015;52:65ŌĆō72.
13. Bao XY, Duan L, Yang WZ, Li DS, Sun WJ, Zhang ZS, et al. Clinical features, surgical treatment, and long-term outcome in pediatric patients with moyamoya disease in China. Cerebrovasc Dis 2015;39:75ŌĆō81.
14. Lee EH, Yum MS, Ko TS. Risk factors and clinical outcomes of childhood ischemic stroke in a single Korean tertiary care center. J Child Neurol 2012;27:485ŌĆō91.
15. Karasawa J, Touho H, Ohnishi H, Miyamoto S, Kikuchi H. Long-term follow-up study after extracranial-intracranial bypass surgery for anterior circulation ischemia in childhood moyamoya disease. J Neurosurg 1992;77:84ŌĆō9.
16. Kornegay JG. Seizures. Tintinalli JE, Stapczynski JS, Ma OJ, Yealy DM, Meckler GD, Cline DM, editors. Tintinalli's emergency medicine: a comprehensive study guide 8th ed. New York (NY): McGraw-Hill Educations: 2016. p. 1173ŌĆō8.
|
|
|||||||||||||||||||||||||||||||||

 |
 |