 |
 |
AbstractCoronavirus disease 2019 (COVID-19) is associated with a variety of neurologic manifestations. Acute necrotizing encephalopathy (ANE) is a rare, life-threatening complication characterized by rapid deterioration of neurologic status following viral infection, such as influenza or human herpesvirus 6. Since the COVID-19 pandemic, a rise in ANE cases associated with the infectious disease has been reported in adult patients. We present a case of COVID-19-associated ANE in a 9-year-old boy. The patient experienced 3 days of fever and mild respiratory symptoms, followed by lethargy. Magnetic resonance imaging on day 4 showed hyperintensity in the bilateral thalami, midbrain, pons, hypothalamus, and cerebellum, along with some areas of hemorrhage. From the imaging findings, ANE was strongly suspected, leading to the initiation treatment involving a 5-day course of remdesivir and multiple immunomodulator therapies, including high-dose corticosteroids, intravenous immunoglobulin, tocilizumab, and 10 cycles of therapeutic plasma exchange. Subsequently, the patient gradually improved, experiencing only minor neurological sequelae and showing favorable radiologic improvement. In COVID-19-infected patients presenting neurologic symptoms, it is crucial to promptly suspect and investigate unexplained encephalopathy using neuroimaging. Early administration of immunomodulator therapy is vital for the diagnosis and optimizing clinical outcomes.
IntroductionCoronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), is primarily characterized by respiratory symptoms. However, there have been reports of various neurological manifestations, ranging from mild symptoms, such as anosmia, ageusia, and headache, to severe life-threatening manifestations, such as status epilepticus, severe encephalopathy, stroke, Guillain-BarrГ© syndrome, acute fulminant cerebral edema, and acute disseminated encephalomyelitis (1,2).
Acute necrotizing encephalopathy (ANE) is a rare, life-threatening encephalopathy that is more commonly observed in children and often preceded by viral infections, such as influenza viruses or human herpesvirus 6. ANE is characterized by symmetrical brain lesions involving both thalami, cerebral white matter, and brainstem (3,4). The prognosis is generally poor because the disease is associated with rapid cognitive deterioration, high mortality rates, and severe neurologic sequelae. Despite the rarity of ANE, the COVID-19 pandemic led to an increasing number of reports highlighting the disease in both adults and children (5,6).
Herein, we present a case of pediatric COVID-19-associated ANE, which was successfully treated with multiple immunomodulator therapies. This study was approved by the institutional review board of Asan Medical Center (IRB no. S2023-1050-0001).
CaseA 9-year-old boy presented to an outside emergency department with drowsiness. Four days before the mental change, he was initially diagnosed with COVID-19 by reverse transcriptase polymerase chain reaction (RT-PCR). He developed a fever, cough, and nasal congestion for the next 3 days. Neither serum biochemistry examination nor brain computed tomography revealed specific abnormalities. On the next day, the patient was transferred to our emergency department for further evaluation and management. At the time of arrival, it was reported that he had had 2 episodes of seizure prior to the abovementioned seizure, the first being a simple febrile seizure at 10 months old. After experiencing his second seizure at 18 months, he was diagnosed with unidentified viral encephalitis and received an anti-epileptic drug until 6 years of age. No further convulsions occurred. The initial vital signs were as follows: blood pressure, 100/53 mmHg; heart rate, 67 beats/minute; respiratory rate, 17 breaths/minute; temperature, 36.3 в„ғ; oxygen saturation, 99% on room air, and a Glasgow Coma Scale score of 12 (eye opening 3, verbal response 4, and motor response 5 [only responsive to loud noises and painful stimuli]). He had a stiff neck and showed a positive Brudzinski sign. His muscle tone was normal with no clonus. No other abnormalities were revealed on further physical and neurological examinations.
White blood cell count was 2,500/ВөL with an 820/ВөL lymphocyte count. No abnormalities were evident in terms of electrolytes, coagulation, serum ammonia, lactate, or inflammatory markers, such as C-reactive protein and erythrocyte sedimentation rate. Computed tomography showed hypodense lesions in both thalami and pons without evidence of hemorrhage or edema (Fig. 1). The cerebrospinal fluid (CSF) findings were as follows: opening pressure, 8 cmH2O (reference value, 11.5-28.0 cmH2O (7)); white blood cells, 78/ОјL (lymphocytes, 46%); protein, 59.1 mg/dL; and glucose, 75 mg/dL. A CSF film array panel (FilmArray Meningitis/Encephalitis [ME] Panel, BioFire DiagnosticsВ®) was negative for the following organisms: Escherichia coli, Haemophilus influenzae, Listeria monocytogenes, Neisseria meningitidis, Streptococcus agalactiae, S. pneumoniae, cytomegalovirus, enterovirus, herpes simplex viruses 1 and 2, human herpesvirus 6, human parechovirus, varicella-zoster virus, Cryptococcus neoformans, and C. gattii. Given the CSF findings, vancomycin, cefotaxime, and acyclovir were empirically administered. Additionally, a 5-day course of remdesivir, immunoglobulin (400 mg/kg/day), and methylprednisolone (1 mg/kg/day) was initiated for severe COVID-19.
On day 2, his mental status deteriorated to a Glasgow Coma Scale score of 6 (eye opening 1, verbal response 1, and motor response 4). He was transferred to the pediatric intensive care unit, and started receiving high-dose methylprednisolone pulse therapy (30 mg/kg/day for 5 days). On day 3, we noted magnetic resonance imaging findings indicative of COVID-19-associated ANE (Fig. 2). Consequently, 1 dose of tocilizumab, a monoclonal antibody against interleukin 6 receptors, was added as an immunomodulator, and therapeutic plasma exchange (TPE) was considered. On day 7, electroencephalography showed continuous slow wave activity without an improvement in the mental status. Consequently, TPE was initiated. After the first TPE session on day 8, the patient began to respond to voice or gentle stimuli, and he remained awake for a few hours each day. He became able to say simple words, make eye contact, and move his legs spontaneously on day 10. By day 11, his overall neurological status improved, although he remained disoriented most of the time.
By day 21, the patient completed 10 TPE sessions, commenced oral intake, and became able to walk with support and talk fluently. On day 24, a follow-up magnetic resonance imaging showed a significant reduction in the hyperintensities (Fig. 3). He was mostly alert at this stage although he occasionally experienced slight disorientation. Muscle power in all extremities was graded a 3-4 on the Medical Research Council scale. He required swallowing therapy due to pharyngeal incoordination and mild dysphagia. On day 35, he was transferred to another hospital to continue rehabilitation. At this time, we noted negative findings of serum myelin oligodendrocyte glycoprotein antibody and anti-N-methyl-D-aspartate receptor antibody as markers of autoimmune encephalitis and paraneoplastic causes, respectively. Chromosome analysis, chromosomal microarray, and whole-genome sequencing showed a mutation in the RANBP2 gene (c.1754C>T, p.Thr585Met). His family underwent genetic counseling prior to this, and he was referred to the pediatric infection department for consultation regarding antiviral vaccination to prevent the recurrence of ANE.
DiscussionANE is a rare acute encephalopathy primarily affecting children, characterized by rapid neurological deterioration and a poor prognosis following viral infection. The diagnosis is typically based on a comprehensive clinical assessment, laboratory findings, and neuroimaging (8). Seizures represent the most common symptom. Other associated features include intracerebral hemorrhage, coma, cerebral edema, vomiting, diarrhea, shock, and multi-organ failure (9,10). A key diagnostic feature is bilateral symmetric thalamic involvement. It is characterized by symmetric and multifocal lesions in the thalami, putamen, cortical and cerebellar white matter, and tegmentum (11). Laboratory findings often include elevated liver enzymes, thrombocytopenia, and hyperammonemia (12). CSF analysis usually shows normal cell counts and increased protein concentrations (4,9).
The presumed pathophysiology of COVID-19-associated ANE is disruption of the blood-brain barrier, triggered by immune-mediated cytokine storm, rather than direct viral invasion into the central nervous system (13). As observed in association with ANE, elevated concentration of cytokines, such as tumor necrosis factor alpha and interleukin-6, has been shown to contribute to blood-brain barrier disruption and to play a role in the overall pathogenesis of the condition (9). Since the onset of the COVID-19 pandemic, there has been a notable increase in the number of cases of COVID-19-associated ANE, along with other neurological complications, in patients with COVID-19 (5,6). The entry of SARS-CoV-2 into the human cells is believed to occur through interactions between viral spike proteins and angiotensin-converting enzyme 2 receptors. Expression of the receptors has been observed in various cells of the central nervous system, including neurons, glial cells of the cerebrum, cerebellum, brainstem, retina, and olfactory mucosa (14). COVID-19 patients have exhibited evidence of SARS-CoV-2 in their CSF, detected through various methods, such as RT-PCR, immunochemistry, and electron microscopy analysis of brain cell autopsies (15). However, CSF testing using RT-PCR has only yielded positive results in 1 case of ANE after repeated sampling (16). Another case report of COVID-19-associated ANE detailed the use of CSF histopathological analysis and metagenomic next-generation sequencing, which is generally more sensitive at detecting infectious pathogens than RT-PCR, but no pathogenic nucleic acids were detected (17). These findings support the abovementioned presumed immune-mediated pathophysiology.
This is the first case report of COVID-19-associated ANE in a pediatric patient confirmed to have a RANBP2 mutation in Korea. A missense mutation in RANBP2 (c.1880C>T: p.Thr585met) is associated with familial or recurrent ANE (ANE1) (18). RANBP2 is a nuclear pore protein that may contribute to the pathogenesis of ANE1, including the regulation of proteostasis, chemokine signaling, intracellular metabolism, and mitochondrial distribution (13). RANBP2 mutations create a predisposition to ANE1 but are inherited in an autosomal dominant manner with incomplete penetrance (18). Additional genetic and environmental triggers are likely involved.
Considering the potential contribution of systemic inflammation and cytokine storm to neurologic injury, therapeutic options for ANE may be immunomodulatory, such as corticosteroids and intravenous immunoglobulin, tocilizumab, and TPE despite a need for further establishment of benefits of the interventions (19,20). We attempted multiple immunomodulator therapies for the patient, indicating uncertainty about the effectiveness of a specific immunomodulator. A prior Japanese retrospective study indicated that the administration of corticosteroids within 24 hours after ANE onset produced better outcomes in patients without brainstem lesions (19). Therefore, the timing of an immunomodulator therapy could be crucial for the prognosis. Early recognition of ANE in patients presenting with altered mental status or coma, allowing for prompt diagnostic neuroimaging, will likely improve the outcomes.
NotesAuthor contributions Conceptualization: Ock-Bin Im and Won Kyoung Jhang Methodology: all authors Software: not applicable Validation: not applicable Formal analysis: not applicable Investigation: Ock-Bin Im and Won Kyoung Jhang Resources: Ock-Bin Im Data curation: Ock-Bin Im Writing-original draft: all authors Writing-review and editing: all authors Visualization: Ock-Bin Im and Won Kyoung Jhang Supervision: Won Kyoung Jhang Project administration: Won Kyoung Jhang Funding acquisition: not applicable Approval of final manuscript: all authors Fig.В 1.Computed tomography scans of the patient on day 1, showing hypodense lesions (arrows) in both thalami (A) and pons (B). 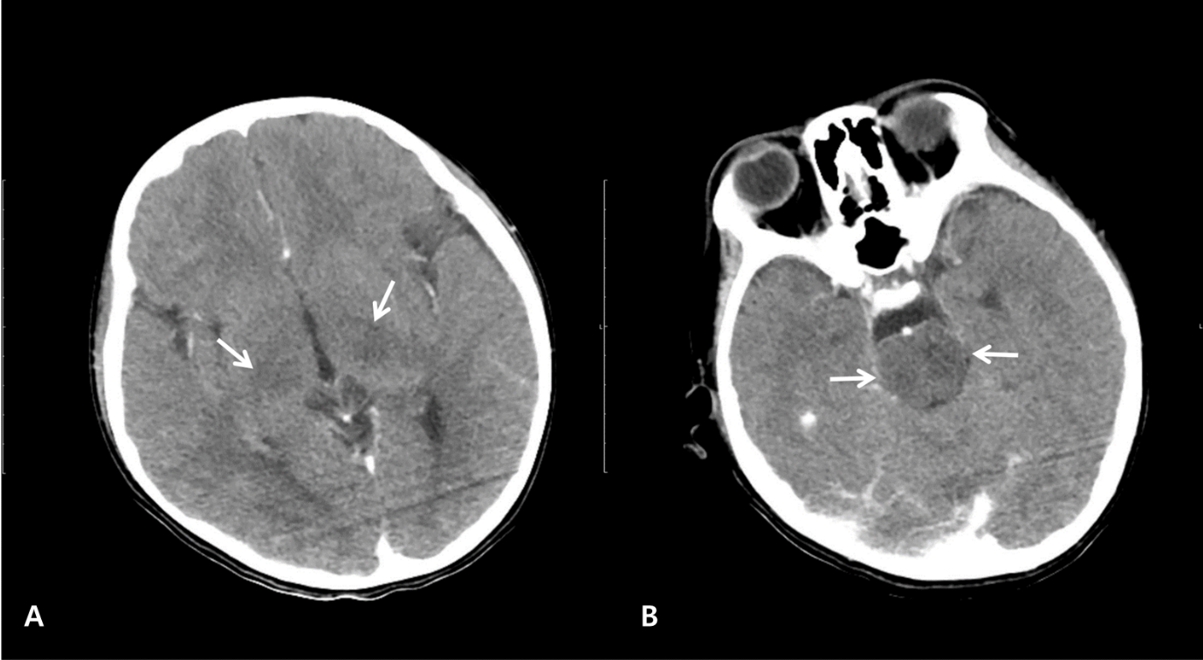
Fig.В 2.Magnetic resonance imaging on day 3. The fluid-attenuated inversion recovery images show hyperintense lesions of both thalami (arrows, A), midbrain (image not shown), pons (arrows, B), hypothalamus (image not shown), and cerebellum (arrowhead, B). The diffusion-weighted images show restrictive lesions in both thalami (arrows, C), pons (arrows, D), and cerebellum (arrowhead, D). The susceptibility-weighted images show hypointense hemorrhagic foci in both thalami (arrows, E), pons (arrows F), and hypothalamus (image not shown). 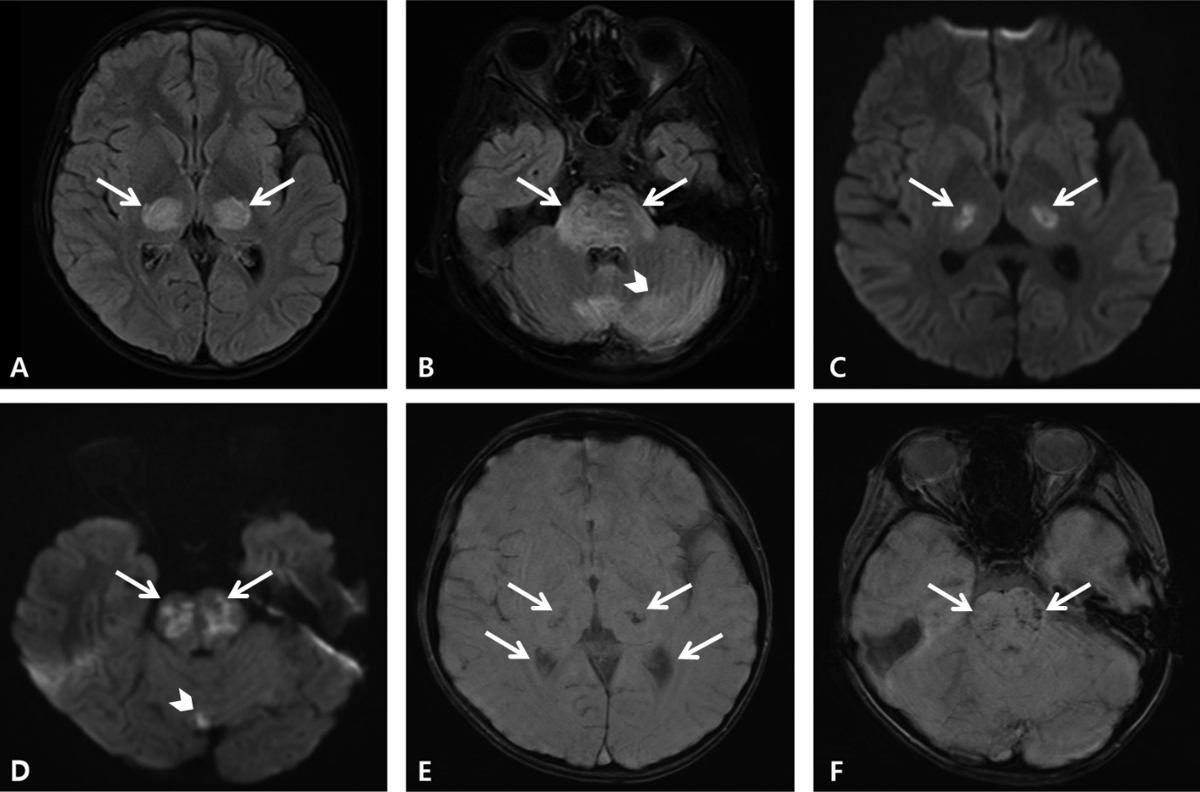
Fig.В 3.Follow-up magnetic resonance imaging on day 24. The fluid-attenuated inversion recovery (A) and T2-weighted (B) images show nearly eliminated hyperintensities involving both thalami (arrows, A), as well as the midbrain (image not shown), pons (arrows, B), hypothalamus, and left cerebellum (images not shown). The susceptibility-weighted images show multifocal hemorrhagic foci in the pons (arrows, C), midbrain (image not shown), and both thalami (arrows, D). 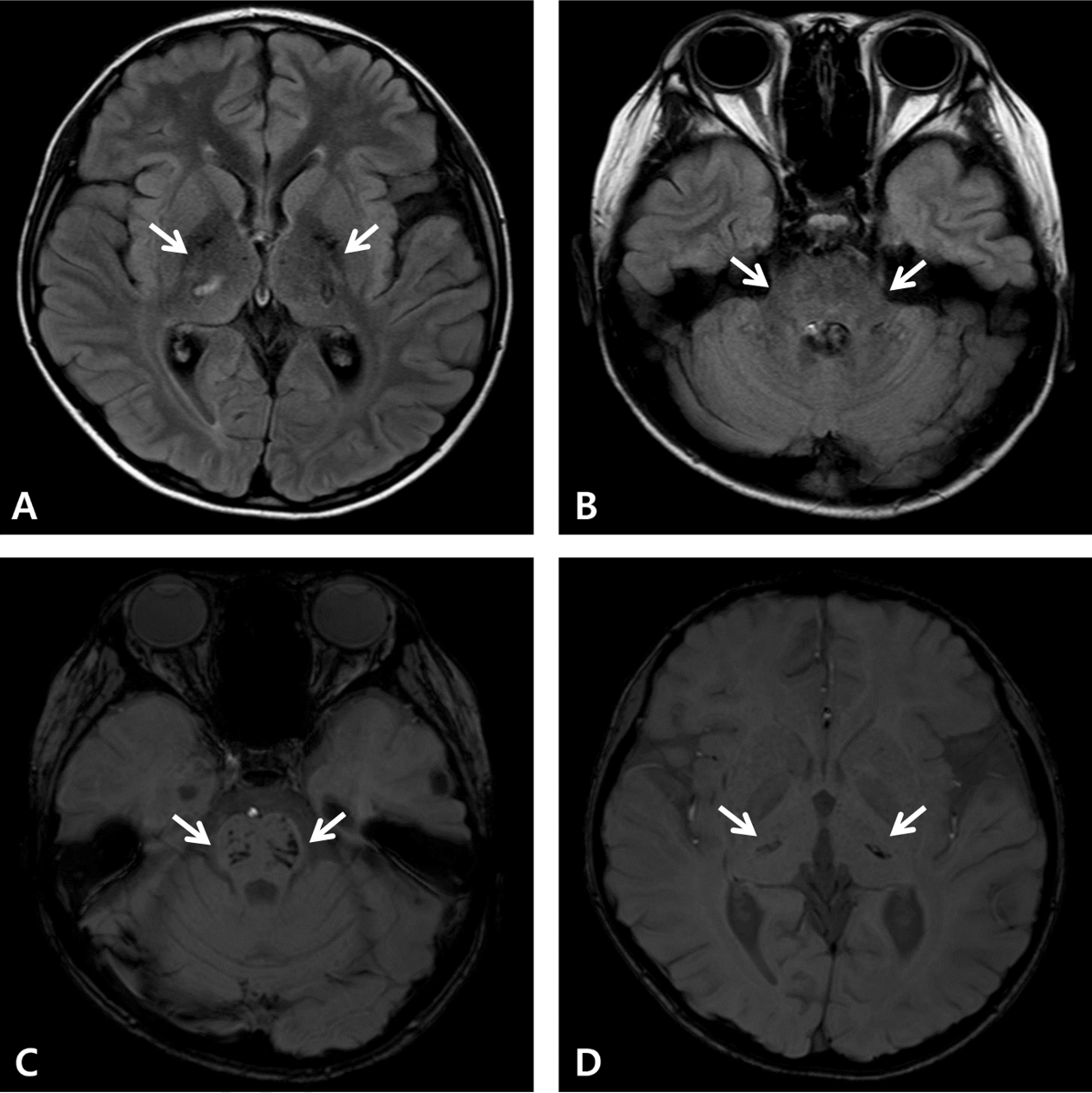
References1. Mao L, Jin H, Wang M, Hu Y, Chen S, He Q, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol 2020;77:683вҖ“90.
2. LaRovere KL, Riggs BJ, Poussaint TY, Young CC, Newhams MM, Maamari M, et al. Neurologic involvement in children and adolescents hospitalized in the United States for COVID-19 or multisystem inflammatory syndrome. JAMA Neurol 2021;78:536вҖ“47.
3. Vanjare HA, Selvi BT, Karuppusami R, Manesh A, Gunasekaran K, Prabhakar AT, et al. Clinical and radiologic findings of acute necrotizing encephalopathy in young adults. AJNR Am J Neuroradiol 2020;41:2250вҖ“4.
4. Mizuguchi M. Acute necrotizing encephalopathy of childhood: a novel form of acute encephalopathy prevalent in Japan and Taiwan. Brain Dev 1997;19:81вҖ“92.
5. Poyiadji N, Shahin G, Noujaim D, Stone M, Patel S, Griffith B. COVID-19-associated acute hemorrhagic necrotizing encephalopathy: imaging features. Radiology 2020;296:E119вҖ“20.
6. Lazarte-Rantes C, Guevara-CastaГұГіn J, Romero L, GuillГ©n-Pinto D. Acute necrotizing encephalopathy associated with SARS-CoV-2 exposure in a pediatric patient. Cureus 2021;13:e15018.
7. Avery RA, Shah SS, Licht DJ, Seiden JA, Huh JW, Boswinkel J, et al. Reference range for cerebrospinal fluid opening pressure in children. N Engl J Med 2010;363:891вҖ“3.
8. Mizuguchi M, Ichiyama T, Imataka G, Okumura A, Goto T, Sakuma H, et al. Guidelines for the diagnosis and treatment of acute encephalopathy in childhood. Brain Dev 2021;43:2вҖ“31.
9. Shukla P, Mandalla A, Elrick MJ, Venkatesan A. Clinical manifestations and pathogenesis of acute necrotizing encephalopathy: the interface between systemic infection and neurologic injury. Front Neurol 2022;12:628811.
10. Wu X, Wu W, Pan W, Wu L, Liu K, Zhang HL. Acute necrotizing encephalopathy: an underrecognized clinicoradiologic disorder. Mediators Inflamm 2015;2015:792578.
11. Wong AM, Simon EM, Zimmerman RA, Wang HS, Toh CH, Ng SH. Acute necrotizing encephalopathy of childhood: correlation of MR findings and clinical outcome. AJNR Am J Neuroradiol 2006;27:1919вҖ“23.
12. Chow CK, Ma CKL. Presentation and outcome of acute necrotizing encephalopathy of childhood: a 10-year single-center retrospective study from Hong Kong. J Child Neurol 2020;35:674вҖ“80.
13. Levine JM, Ahsan N, Ho E, Santoro JD. Genetic acute necrotizing encephalopathy associated with RANBP2: clinical and therapeutic implications in pediatrics. Mult Scler Relat Disord 2020;43:102194.
14. Lou JJ, Movassaghi M, Gordy D, Olson MG, Zhang T, Khurana MS, et al. Neuropathology of COVID-19 (neuro-COVID): clinicopathological update. Free Neuropathol 2021;2:2.
15. DosSantos MF, Devalle S, Aran V, Capra D, Roque NR, Coelho-Aguiar JM, et al. Neuromechanisms of SARS-CoV-2: a review. Front Neuroanat 2020;14:37.
16. Virhammar J, Kumlien E, FГӨllmar D, Frithiof R, Jackmann S, SkГ¶ld MK, et al. Acute necrotizing encephalopathy with SARS-CoV-2 RNA confirmed in cerebrospinal fluid. Neurology 2020;95:445вҖ“9.
17. Rettenmaier LA, Abdel-Wahed L, Abdelmotilib H, Conway KS, Narayanan N, Groth CL. COVID-19-associated necrotizing encephalopathy presenting without active respiratory symptoms: a case report with histopathology. J Neurovirol 2022;28:172вҖ“6.
18. Neilson DE, Adams MD, Orr CM, Schelling DK, Eiben RM, Kerr DS, et al. Infection-triggered familial or recurrent cases of acute necrotizing encephalopathy caused by mutations in a component of the nuclear pore, RANBP2. Am J Hum Genet 2009;84:44вҖ“51.
|
|
|||||||||||||||||||||||||||||||||||

 |
 |